Database FAQ
Use the links below to jump to the answer you're looking for.
- Q1: I've forgotten my database username or password - what should I do?
- Q2: How do I change a follow-up's due date on the database?
- Q3: What do I do if a patient did not attend (DNA) their follow-up visit, or if they've been discharged?
- Q4: What is the 21-day edit window and how do I close it?
- Q5: How do I enter drug doses?
- Q6: How do I indicate a dose change for a biologic or conventional therapy?
- Q7: How do I report an Adverse Event (AE), or a Serious Adverse Event (SAE) such as death?
- Q8: How do I enter an Event of Special Interest (ESI) form?
Q1: I've forgotten my database username or password - what should I do?
If you've forgotten your password, you can use the Forgotten Password page on our database. Enter your username and a new password will be emailed to you. If the email doesn't arrive within a couple of minutes, please check your spam/junk folder.
- Passwords must include a number and a special character (such as !"£$%^&*()_+) - please copy and paste the new password from the email to ensure it is entered correctly.
If you've forgotten your username, and you've tried using your email address, please get in touch and we'll be able to help. If you provide us with your email address we will be able to find your account and tell you your username. Once you know your username, follow the process above if you've also forgotten your password.
Q2: How do I change a follow-up's due date on the database?
If a patient's follow-up dates on the BADBIR database don't match the clinic dates for when they'll be seen, they can be brought back into line. Six months before a follow-up is due, you'll see an Edit button to the left of the follow-up. Click Edit, change the due date, then click Update.
- If you accidentally change the due date to a date more than six months in the future, you won't be able to change it back.
If all of the due dates are out of sync with the patient's clinic visit dates, please contact the office and we'll be able to change them all at once.
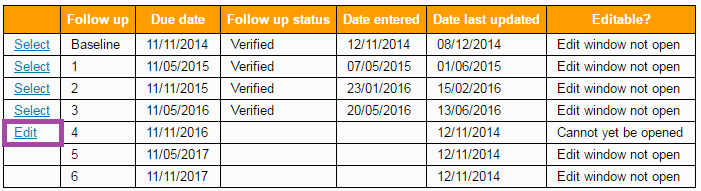
Q3: What do I do if a patient did not attend (DNA) their follow-up visit, or if they've been discharged?
If a patient does not attend their appointment for a follow-up visit, you may not have any data to enter onto the BADBIR database. In this case, it's best to let us know using the Feedback section so we can update the follow-up status to Missed / Data cannot be recorded. This helps to keep the database tidy, as it is clear when the patient has not attended, and it's removed from your list of overdue follow-ups.
Of course, if there is information from the notes applicable to the time period covered by the follow-up, please enter this onto the database. Remember to leave a note in the Feedback section to say that the patient didn't attend, so we don't raise queries for the other missing data.
For patients who do not attend over a longer period or have been discharged, our standard practice is to mark one year's worth of follow-ups as Missed / Data cannot be recorded, so that we get an annual update on the status of the patient in case they have been referred to dermatology again. We also change the patient's status to Not attending so that it is clear to our admin staff that queries shouldn't be raised for any patient questionnaires on follow-ups that have been entered.
Q4: What is the 21-day edit window and how do I close it?
The 21-day edit window is the period of time that you are allowed to add and change data within a follow-up. Once this period ends, you will be able to view the data that's been entered, but you won't be able to change it. If you've noticed a mistake and the edit window is closed, please contact us and we'll be able to help.
The 21-day edit window begins when you click Select to open a follow-up for the first time, and ends after 21 days unless it is manually closed. Once closed, it is transferred to an inbox monitored daily by the BADBIR admin staff, who will check the information entered in the follow-up and raise any data queries if necessary. See our Avoiding Data Queries page for more information on how to make sure you incur as few queries as possible!
Manually closing the edit window means the follow-up will be checked sooner, usually within one working day of closing it. This might mean the patient notes are still available by the time any queries are raised, which could save time in solving the queries.
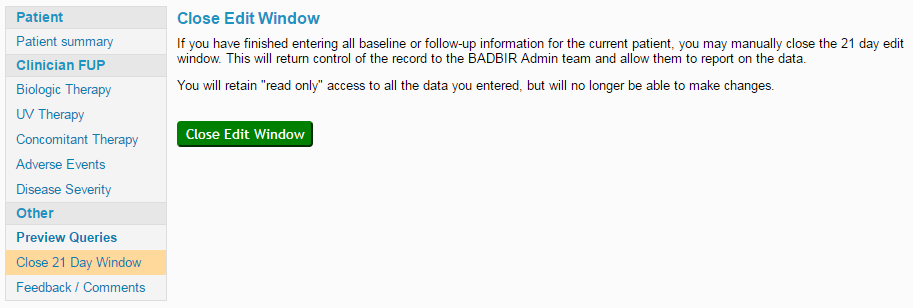
- Remember the Preview Queries section can help to reduce your queries before you even close the edit window.
Q5: How do I enter drug doses?
Some of the biologic drugs we register for have large gaps between scheduled doses. This means it's much more significant if a patient misses a dose, compared to missing a dose in a weekly schedule for example. This means we collect individual dose dates so we can keep track of any missed doses. We do this for the main drugs listed below:
- Stelara (Ustekinumab) - usually 45mg or 90mg 12-weekly (following the loading doses)
- Cosentyx (Secukinumab) - usually 300mg 4-weekly (following the loading doses)
We also collect individual dose dates for Remicade (Infliximab), Remsima (Infliximab biosimilar) and Inflectra (Infliximab biosimilar). These are usually given in 5mg/kg doses at 8-week intervals.
There's an extra step in the data entry process for these drugs, compared with other biologic and conventional therapies. Once the main details have been entered (drug name, start date, dose and frequency), a section will appear to add the dosage and the date of administration. The first dose is entered for you as the start date of the drug. Please enter any other administration dates that fall within the current follow-up period.
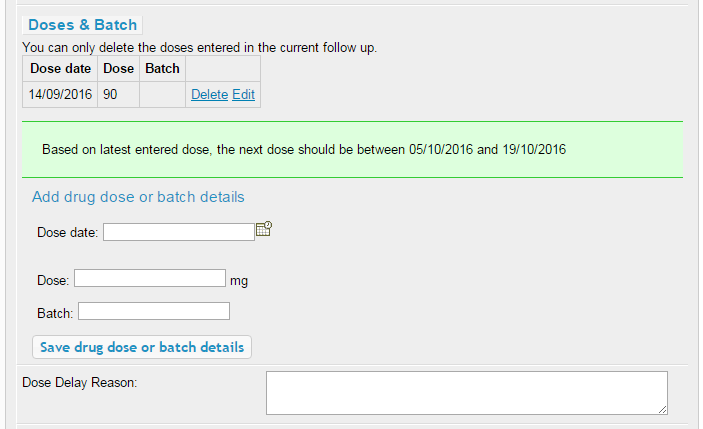
The green banner shows the date range we expect the next dose to fall within, based on the drug's normal dosing schedule. If the patient has missed a dose for whatever reason, or the doses have deviated from the normal schedule, the database may prompt you to provide a reason for this. Please enter the reason into the Feedback section, which will be reviewed by our admin staff and should prevent a query from being raised in this regard.
Sometimes the actual dates of administration aren't recorded in the patient's notes. We ask you to confirm the following:
"To the best of your knowledge, did the patient deviate from the normal schedule?"
It's important that we keep track of this in case of any adverse events; the length of time since the last administration can be significant.
- Remember to enter this into the Feedback section to avoid a query being raised - drug doses are commonly missed and queried.
Q6: How do I indicate a dose change for a biologic or conventional therapy?
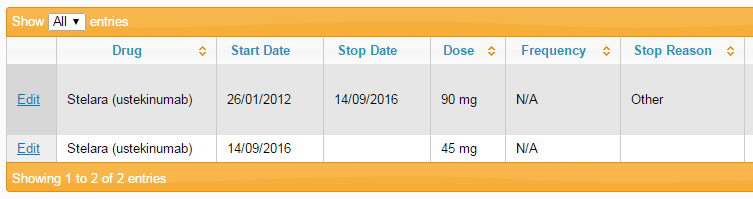
To represent a dose change on the BADBIR database, a stop date is entered for the current course, and a new course is entered starting from that date. The stop reason for the original course can be set as Other, with the text "Dose change" entered in the details box, as shown below.
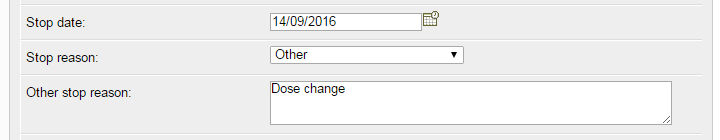
Once you've entered the stop details, save the page, then click the Add New button and enter the details of the new dosage. Save this page, and you should see something similar to the image below:
Q7: How do I report an Adverse Event (AE), or a Serious Adverse Event (SAE) such as death?
For all of our information on Adverse Events and Serious Adverse Events, please see the section on our information page:
Recording an AE or SAEQ8: How do I enter an Event of Special Interest (ESI) form?
To see information on entering ESIs, please head over to the section on the Adverse Events Information page:
Entering ESI forms